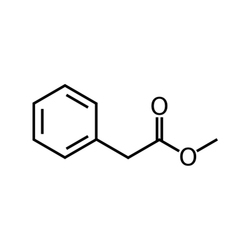
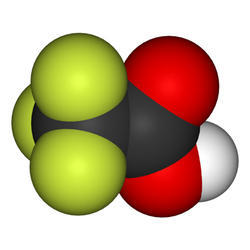
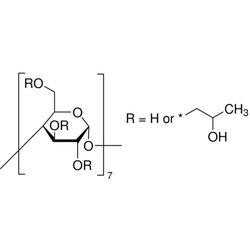
Speciality Chemicals
Bromoform
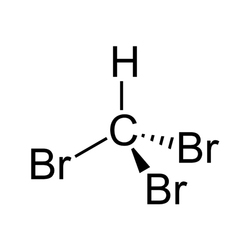
Leading Wholesale Trader of bromoform, iodoform, methyl phenylacetate, trifluroacetic acid, hydroxypropyl beta cyclodextrin and 4-methoxy phenacyl bromide from Mumbai.Bromoform (CHBr3) is a brominated organic solvent, pale yellow liquid at room temperature, with a high refractive index, very highdensity, and sweet odor is similar to that of . It is a trihalomethane, and is one of the four haloforms, the others beingfluoroform, , and iodoform. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating with aluminum bromide. Currently its main use is as a laboratory reagent.
USesOnly small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, and flame retardant, but now it is mainly used as a laboratory reagent, for example as an extraction solvent.Bromoform's high density makes it useful for separation of minerals by density. When two samples are mixed with bromoform and then allowed to settle, the top layer will contain minerals lighter than bromoform, and the bottom layer will contain heavier minerals. Slightly less dense minerals can be separated in the same way by mixing the bromoform with a small amount of a less dense and fully miscible solvent
Product Details| Minimum Order Quantity | 1 Kilogram |
| Formula | CHBr3 |
| CAS Number | 75-25-2 |
| Molar Massa | 252.73 g.mol-1 |
| Density | 2.89 g mL-1 |
| Melting Pointr | 4 to 16 Deg C |
| Boiling Point | 147 to 151 Deg C |
Iodoform
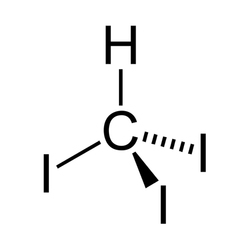
Iodoform is the organoiodine compound with the formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as the smell of hospitals, where the compound is still commonly used) and, analogous to , sweetish taste. It is occasionally used as a disinfectant. It is also known as tri-iodomethane, carbon triiodide, and methyl triiodide.
usesIodoform is primarily used to treat minor skin conditions due to its antiseptic properties. It is also used in various human and animal disinfectant products, and in polarizing films for liquid crystal display or LCD chemicals
Product Details| Minimum Order Quantity | 1 Kilogram |
| Formula | CHI3 |
| CAS Number | 75-47-8I |
| Molar Massa | 393.73 g.mol-1 |
| Density | 4.008 g mL-1 |
| Melting Pointr | 119 Deg C |
| Boiling Point | 218 Deg C |